by Caleb Horst | Oct 23, 2020 | In The Lab
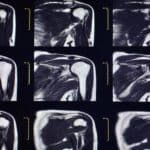
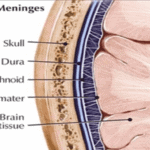
Asthma, emphysema, COVID-19 and other diseases lead to changes in conducting pulmonary volume, viscoelasticity, and air flow distribution. To study these effects, Prof. Mona Eskandari at UC Riverside partnered with CellScale to design and build custom equipment....
by Caleb Horst | Jul 28, 2020 | In The Lab
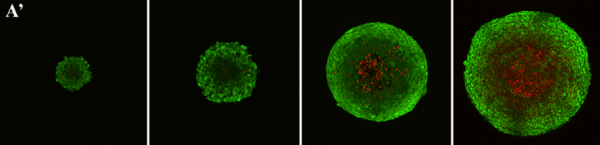
Tissue spheroids are a growing pre-clinical tool for drug development. Succeeding in this application will require quality, repeatability, and standardization. The MicroTester is perfectly suited for mechanical compression of tissue spheroids. In a recent paper Elena...
by Caleb Horst | May 14, 2020 | In The Lab
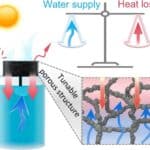
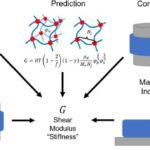
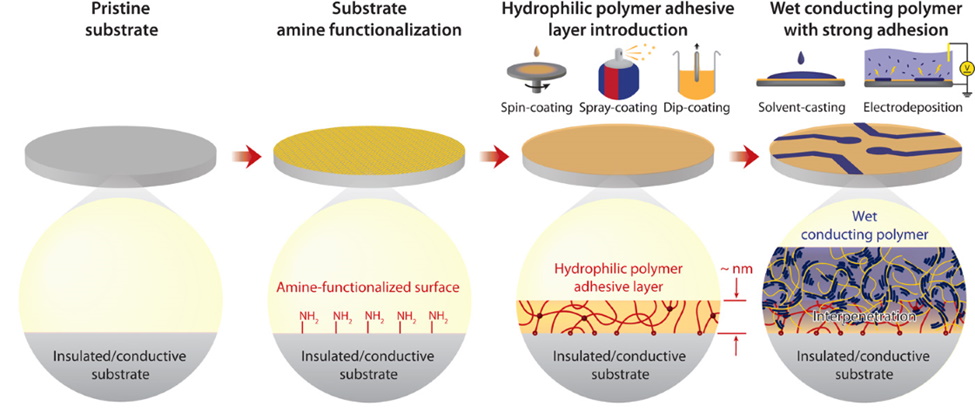
Conducting polymers serve as an interface between electrodes and biological organisms in bioelectronic devices. Some examples are poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS), polypyrrole (PPy), and polyaniline (PAni) which have favorable...
by Caleb Horst | Apr 14, 2020 | In The Lab
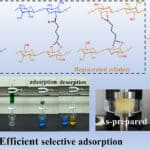
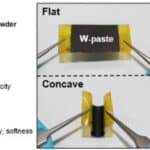
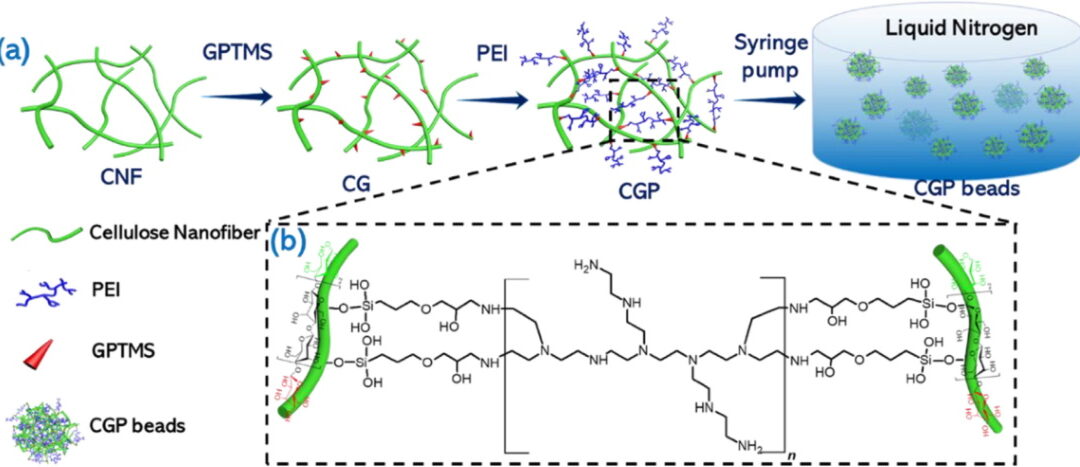
Heavy metals are serious pollutants in wastewater that are discharged to the environment. Although there are various methods to remove them, the most common method is commercial powder absorbents. These have poor recyclability (which can lead to secondary...
by Caleb Horst | Apr 7, 2020 | In The Lab
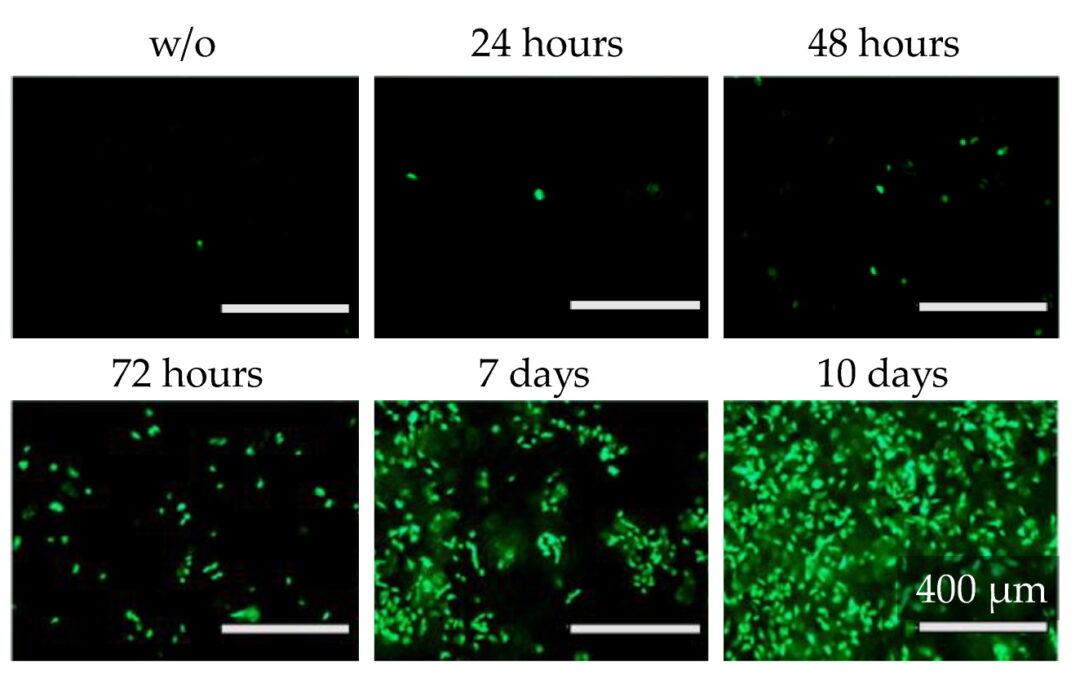
New in vitro models using human cells are increasingly being used to replace animal testing for drug development. Freshly isolated human hepatocytes are ideal in predicting liver toxicity in these models, although they are highly limited and tend to lose their...
by Caleb Horst | Mar 26, 2020 | In The Lab
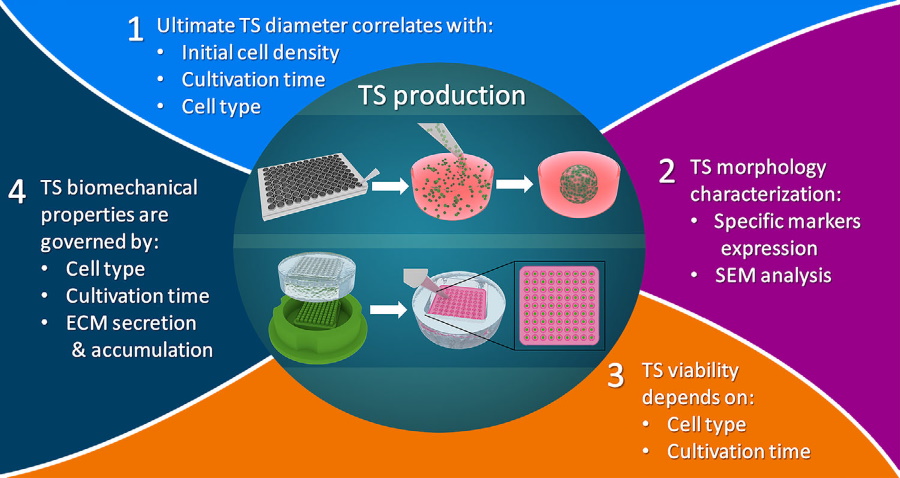
Tissue spheroids (TS) are being increasingly recognized as a powerful tool to create 3D human tissues. This complex cell and matrix composition is formed without scaffolds and can recapitulate the architecture and functional characteristics of native tissue. TS is...